We harness the best people, science, and technologies to provide insights and data for optimal immuno-oncology research and development
With increased complexity in oncology protocol design and time sensitivity, we provide you with a robust adaptive, specialist oncology team with access to the latest science and technology who will optimize the outcome of clinical trials. Precision medicine development in the oncology field requires a flexible and agile specialty laboratory partner who can adjust as the protocol does, ensuring endpoints are captured and enabling studies to move forward with the right data.
Cerba Research can provide integrated specialty testing to your existing central laboratory process.

Scientist to scientist communication.
We understand your challenges, tailoring answers to support your scientific questions.
Dedicated global project management team, and scientific project managers for speciality techniques.
200+ oncology studies in the past 5 years split between solid and liquid tumors in oncology across pharmaceutical and biotech.
Trials in 60 countries with 3200+ sites.
110+ studies ongoing today with 40% Phase I (first-in-human), 39% Phase II, 19% Phase III & 2% Phase IV
Flow cytometry – BD FACS Canto & Lyric as well as Cytek Aurora. Immunophenotyping (including intra-cell markers), MRD detection, receptor occupancy, CAR T cell enumeration/phenotyping and intracellular cytokine detection can be performed. Learn more about our flow cytometry services.
Sequencing – Illumina platforms with numerous oncopanels (broad panel assays), mutational analysis, WES, WGS.
Histopathology – IHC/IF/FISH/ISH with 250+ biomarkers or protocols (Leica, Roche Ventana, RUO/IVD). Read more
Biobanking – Access to a large amount of blocks and growing tumor microarrays. Collaboration with numerous biobanking entities. Read more
Circulating biomarkers – By means of ELISA, ELLA, MSD platforms. Read more
Safety profile – Routine hematology, biochemistry, urinalysis, biochemistry, serology, electrophoresis (sPEP, uPEP) to monitor patient safety. Read more
Liquid biopsies – Growing ctDNA-based panels as detected by NGS. Read more
Digital Imaging – Transforming visual results into quantifiable data with Halo® and Visiopharm® software. Read more
PBMC isolation – Harmonized isolations on a global scale using CPT tubes. Read more
Nanostring – For gene expression and cancer spatial transcriptomics analysis. Read more
Other platforms – Such as Sanger sequencing, fragment analysis, ddPCR, qPCR, caryotyping & FISH.
Custom panels to support oncology therapeutic development using NanoString™ technology
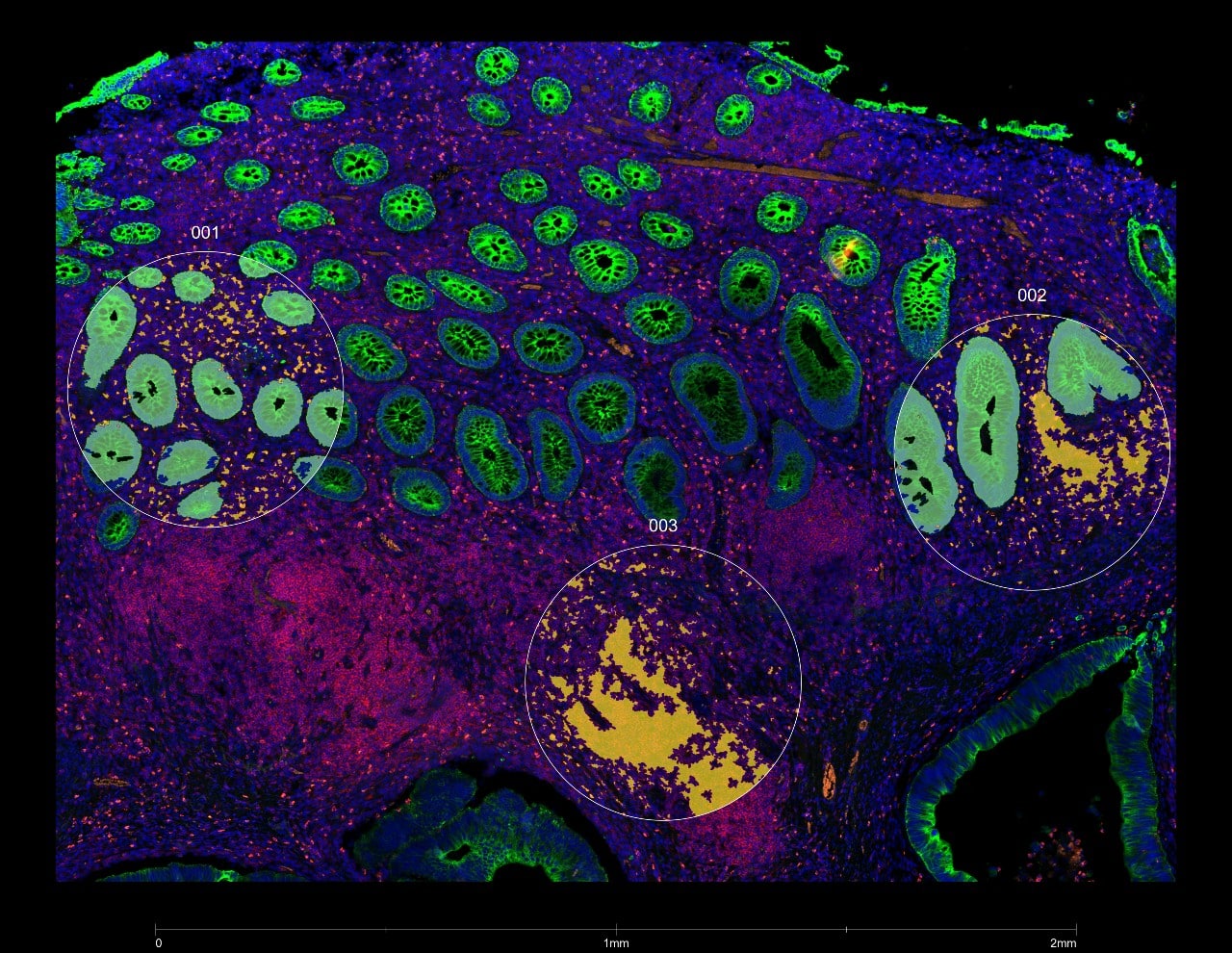
Cerba Research offers tissue omics services for pre–clinical and translational research programs as well as clinical trials. Our flexible solutions allow for quantitative analysis of both proteins and transcripts in whole tissue, either as bulk analysis or spatially in regions of interest and in specific cell populations on tissue sections.
Our laboratory is equipped with the both nCounter® And GeoMx® Nanostring platforms, to provide the most relevant dataset available for each specific request.
Our Latest Oncology Content

Reach out to our experts and see how we can help advance your clinical trials in Immuno-oncology & Oncology
Contact Us
